Abstract
More than a decade on from the first reporting of JAK2 V617F, the Myeloproliferative Neoplasms (MPNs) are circumscribed by an ever more intricate landscape. There is now evidence that MPN is likely the result of combined genetic dysregulation, with mutations in genes involved in the regulation of epigenetic mechanisms increasingly reported. With improved understanding about the molecular complexity of these conditions, newer therapeutic options are starting to emerge. Enhancing our knowledge about the effect of these therapies on the epigenome may help guide their use in the therapeutic setting, and allow improved risk-stratification of patients. 'MAJIC' (ISRCTN61925716) is a UK based randomised study of Best Available Therapy (BAT) versus JAK inhibition (Ruxolitinib) in patients with high risk Polycythaemia vera (PV) or Essential thrombocythaemia (ET), who are resistant to or intolerant of Hydroxycarbamide. Ruxolitinib itself, may also actually have epigenetic capacity, with mutant JAK2 reported to be found in the nucleus, where it can lead to phosphorylation of histone H3 and the arginine methyltransferase PRMT5. This project sought to further investigate the epigenetic effect of Ruxolitinib, firstly in MPN cell lines (UKE-1, SET-2 and HEL) and then in patient samples from the MAJIC clinical trial. The aim was to identify any epigenetic changes potentially associated with the clinical outcome of MPN patients.
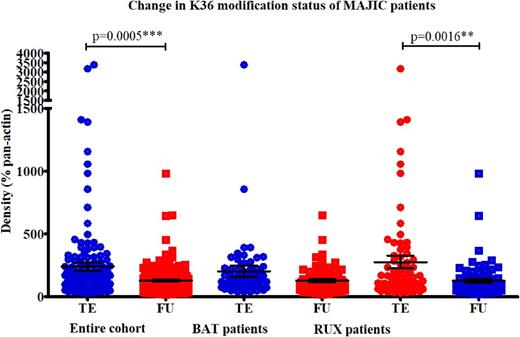
Within cell lines, a clinically relevant dose of Ruxolitinib was established at 100nM, assessing cell viability by Cell titre-glo (CTG®) and then performing western blots utilising antibodies directed at the JAK/STAT pathway. This dose was clinically achievable, being well below the maximum serum concentration seen in a patient. Taking this dose forward, changes across 21 different histone H3 modifications were assessed using the EpiQuik™ histone H3 modification multiplex assay kit in normal bone marrow, and all 3 MPN cell lines. When the data was examined together, most modifications (95%, n=20/21) were increased in Ruxolitinib treated cells, relative to untreated cells, with the values normalised for total H3 present. The six most significant modifications selected for further analysis: methylation marks (mono-, di- and tri-) at lysine 36 and lysine 4. Changes in the levels of these modifications were further assessed by western blotting over a time course mimicking a patient dosing regimen. The results of the histone arrays and western blot validation were positively correlated with an R2 value of 0.7394. The changes in these 6 candidate modifications with treatment was then examined by western blot, in paired trial entry (TE)and follow-up (FU) samples (at a median time of 13 months, range 9-36), from 51 patients enrolled on the MAJIC clinical trial. Methylation at lysine 36 has been associated with transcriptional repression, while at lysine 4, methylation levels are associated with active gene transcription. We had therefore postulated that epigenetic therapy would theoretically increase K36 methylation levels and repress K4 methylation levels, particularly in responsive patients. However, when the cohort was examined both sets of methylation marks were significantly decreased in follow-up samples, compared to trial entry, with densitometry values normalised for pan-actin used as a loading control (K36 p=0.0005, K4 p=0.0095). The decrease in K36 marks was also significant when the Ruxolitinib patients were examined separately, p=0.0016 (see Figure 1). The decrease in K4 marks was significant in the BAT patients when the groups were separated, p=0.017. However, none of the changes in the individual candidate modifications were significantly associated with the therapeutic randomisation of the patients, in either disease group. This suggests that Ruxolitinib was not superior compared to BAT at inducing changes in the histones examined. Finally, although the cohort size was small (n=3/51), high baseline levels of H3K4me2 and H3K4me3 were significantly associated (p<0.0001) with non-response to Ruxolitinib. This finding may suggest that in some patients, JAK inhibitors are unable to overcome these very high baseline levels of transcriptional activation, resulting in reduced clinical efficacy. This would ideally be validated in a larger cohort of patients, but may be an important biomarker that could aid therapeutic decision making.
Harrison: Gilead: Consultancy, Speakers Bureau; Shire: Speakers Bureau; Celgene: Consultancy; Novartis: Honoraria, Research Funding, Speakers Bureau; CTI: Speakers Bureau. Mead: BMS: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau. McMullin: Italfarmaco S.p.A.: Consultancy; Shire, Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal